
(Make sure you visit this, because I have mentioned the Ionic charges along with the images for each element). Main Group Metals: Charges are determined by the group and. But if you want to see the list of ionic charges of all the 118 elements, then visit: Ionic charges of all the elements. Each element has a charge dictated by whether it is a metal (+ charge) or a nonmetal (- charge).

On the other hand, all isolatable particles have charges. These are the ionic charges of first 20 Elements. In contrast Group 7 elements you will find (I will draw an example) it's easier to gain electrons from surroundings because their outer ring is nearly full, so they'll have more electrons than protons and therefore become negatively charged in their ionic state e.g. In this case, one says that the elementary charge is three times as large as the quantum of charge. This therefore means there's one less electron than there are protons hence Na+ charge in it's ionic form.
#As element charge is full#
Atoms like having full rings, so it's just easy if Na 'kicks' out that one electron becoming 2 + 8. See that the outer ring only has 1 electron. (DRAW OUT Na STRUCTURE) - 2 + 8 + 1 = 11. The effective atomic number Z eff, (sometimes referred to as the effective nuclear charge) of an atom is the number of protons that an electron in the element effectively sees due to screening by inner-shell electrons. A trend is to find the charge by looking at the Group on the Periodic. The inner ring only holds 2 all subsequent rings hold 8. When writing formulas for ions and ionic compounds we often need to find the ionic charge.

Remember protons and neutrons are in the nucleus electrons orbit it in rings. If an object has more protons than electrons, then the net charge on the object is positive. Na which has an atomic number of 11 (number of protons and therefore electrons - if both are equal it's a neutral charge). The ionic charge trend in the periodic table is that generally elements on the left form cations and those on the right form anions. Group 2 elements are alkaline Earth Metals with a. (n)- means the opposite. Look at the elements on the periodic table e.g. Group 1 elements are the alkali metals that have a charge of +1. Atoms of the same element with different numbers of neutrons are called. (n)+ means there're more protons (positively charged) than electrons (negatively charged). The effective nuclear charge (often symbolized as Zeff or Z) is the net positive charge experienced by an electron in a multi-electron atom. The charge on the proton and electron are exactly the same size but opposite. Because, remember, gaining electrons gives us a negative charge. By gaining that one electron, they would get a charge now of minus one. Electric charges may be positive or negative in. Charge is a physical property that causes matter to experience a force within an electromagnetic field. So each element in this column would have to gain one electron to become like the noble gas next to it. In the context of chemistry and physics, charge usually refers to electric charge, which is a conserved property of certain subatomic particles that determines their electromagnetic interaction.
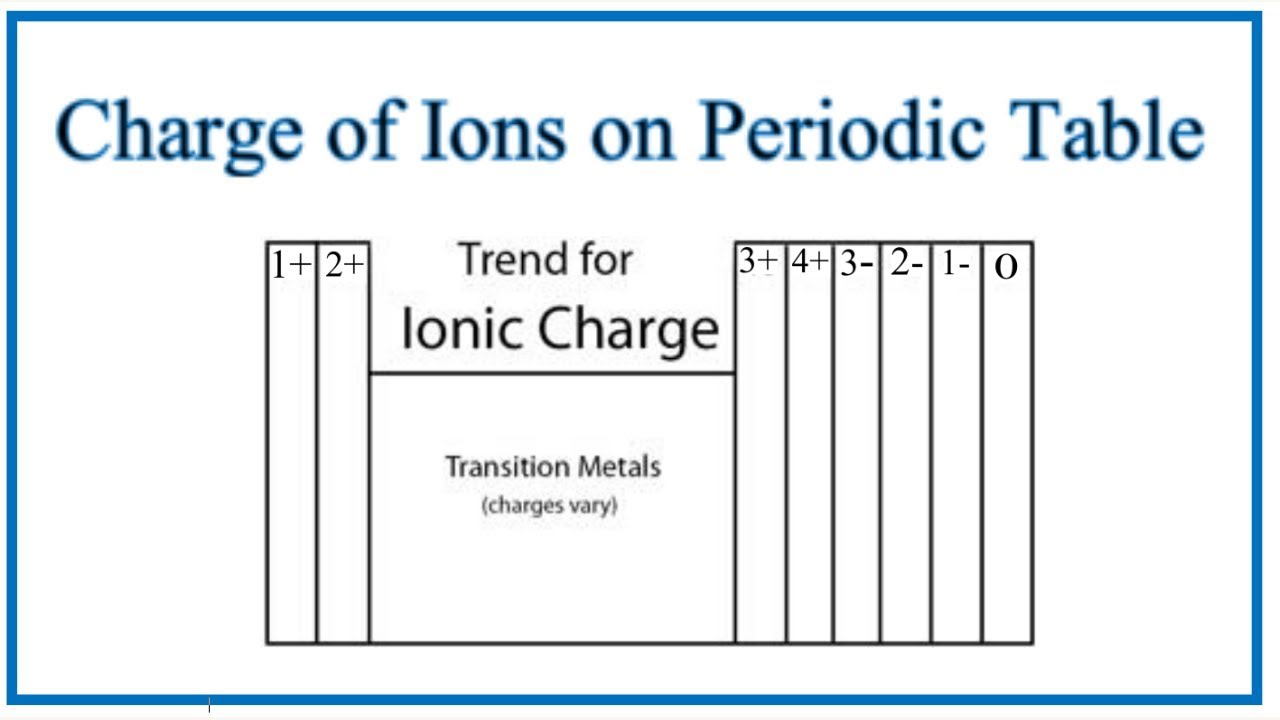

Group 1 elements become 1+, Group 2 becomes 2+, Group 3 becomes 3+, Group 4 becomes 4+, Group 5 becomes 3-, Group 6 becomes 2-, Group 7 becomes 1-, Group 8 is always 0 (inert). Electron chlorine has 17 electrons with neutral to have 18 like are gone again and have to gain one electron. So there's no simple rule for learning the number of electrons an atom may gain or lose in a compound (and using electricity, for example, it's possible to remove electrons well below the valence shell), though the number of electrons in the valence shell is unique.The useful thing is that different elements have been grouped together on the periodic table so that it's easy to see which ones share something in common. " So an element has a specific valence, depending on its group (e.g., C, 4 or Xe, 0), but may have multiple values for valency, such as in $\ce$. It's not simply the column that determines the ionic charge of an element, but also other factors, such as row and with what other elements it's combined.Įpediaa states, "valence refers to the ability of an atom to be combined with another atom whereas valency refers to the maximum number of electrons that an atom can lose or gain in order to stabilize itself.


 0 kommentar(er)
0 kommentar(er)
